FDA authorizes AI tool to predict breast cancer risk
Senior medical analyst Dr. Marc Siegel discusses advancements in artificial intelligence aimed at predicting an individual’s future risk of breast cancer and the increased health risks from cannabis as users age.
NEWYou can now listen to Fox News articles!
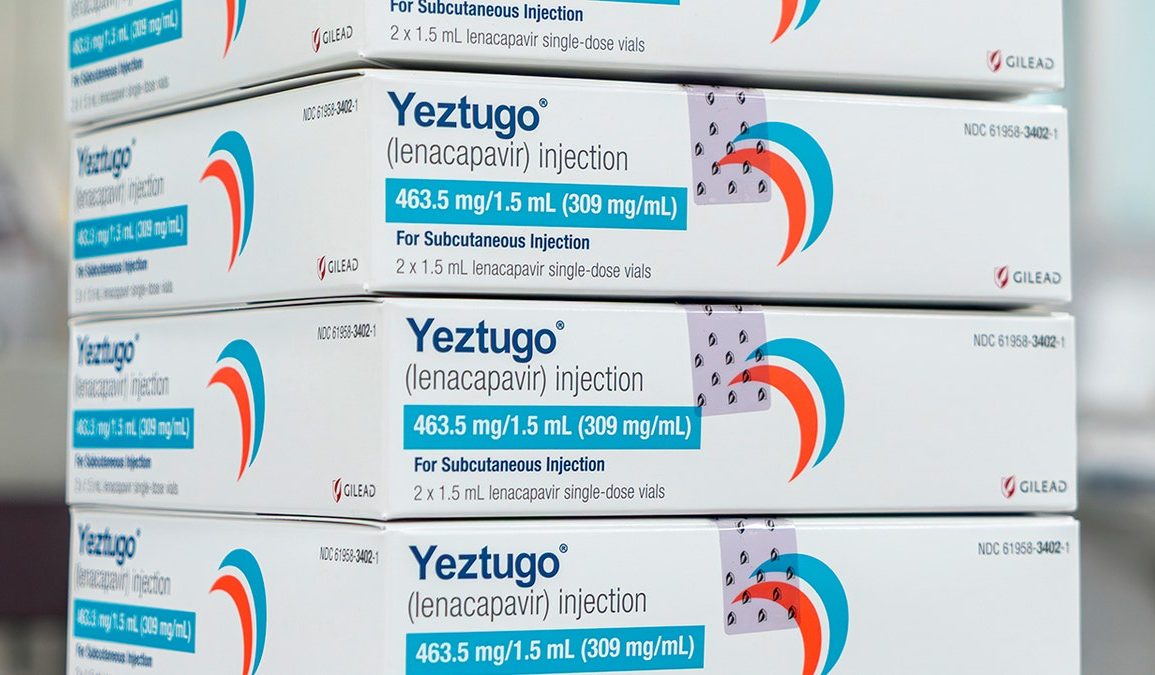
The U.S. Food and Drug Administration (FDA) approved a new, twice-yearly shot — the first and only of its kind — to prevent HIV, the creator of the drug, Gilead Sciences, announced on Wednesday.
Sold under the name Yeztugo, the company’s injectable HIV-1 capsid inhibitor (lenacapavir) reduces the risk of sexually acquired HIV in adults and adolescents.
“This is a historic day in the decades-long fight against HIV,” said Daniel O’Day, chairman and CEO of California-based Gilead Sciences, in a press release.
ALZHEIMER’S DISEASE COULD BE PREVENTED BY ANTIVIRAL DRUG ALREADY ON MARKET
The medicine, which only needs to be administered twice a year, has shown “remarkable outcomes in clinical studies,” as Gilead claims it could transform HIV prevention.
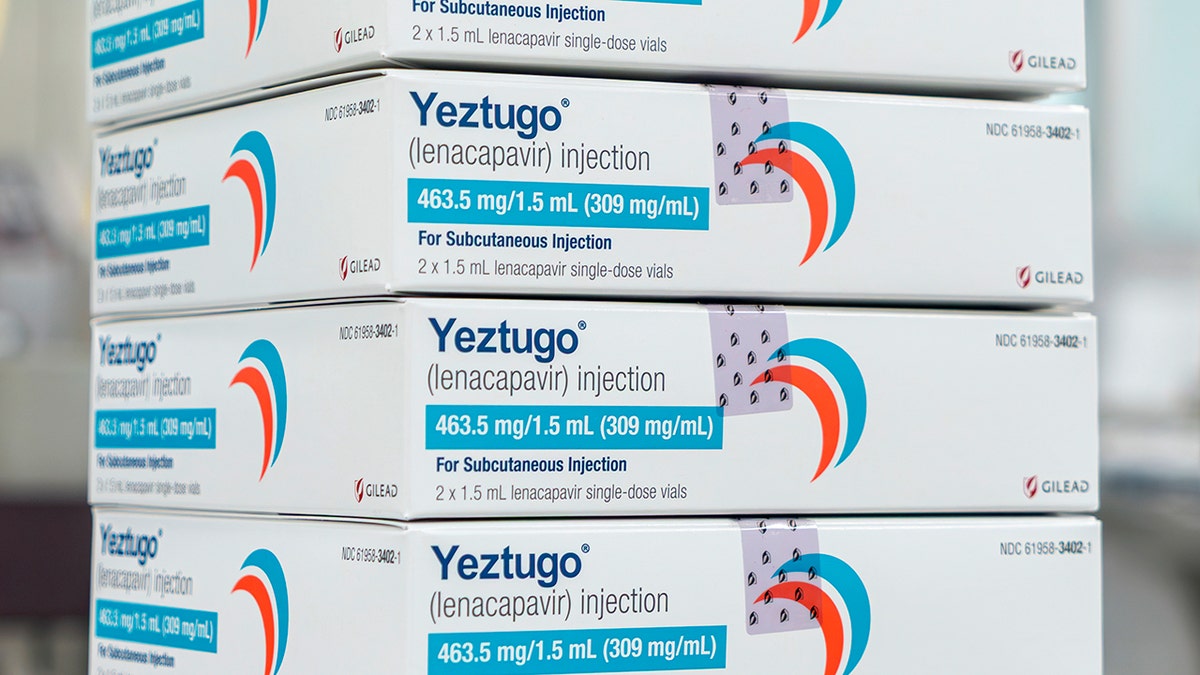
The U.S. Food and Drug Administration has approved a new, twice-yearly shot, Yeztugo, to prevent HIV, the creator of the drug announced on Wednesday. (Gilead Sciences via AP)
The drug is given as an injectable under the skin that the body then slowly absorbs. Individuals must have a negative HIV-1 test prior to starting the treatment.
CLICK HERE TO GET THE FOX NEWS APP
In large trials last year, the drug was not only nearly 100% effective in its prevention of HIV, but proved superior to once-daily oral medication like Truvada, another drug by Gilead.
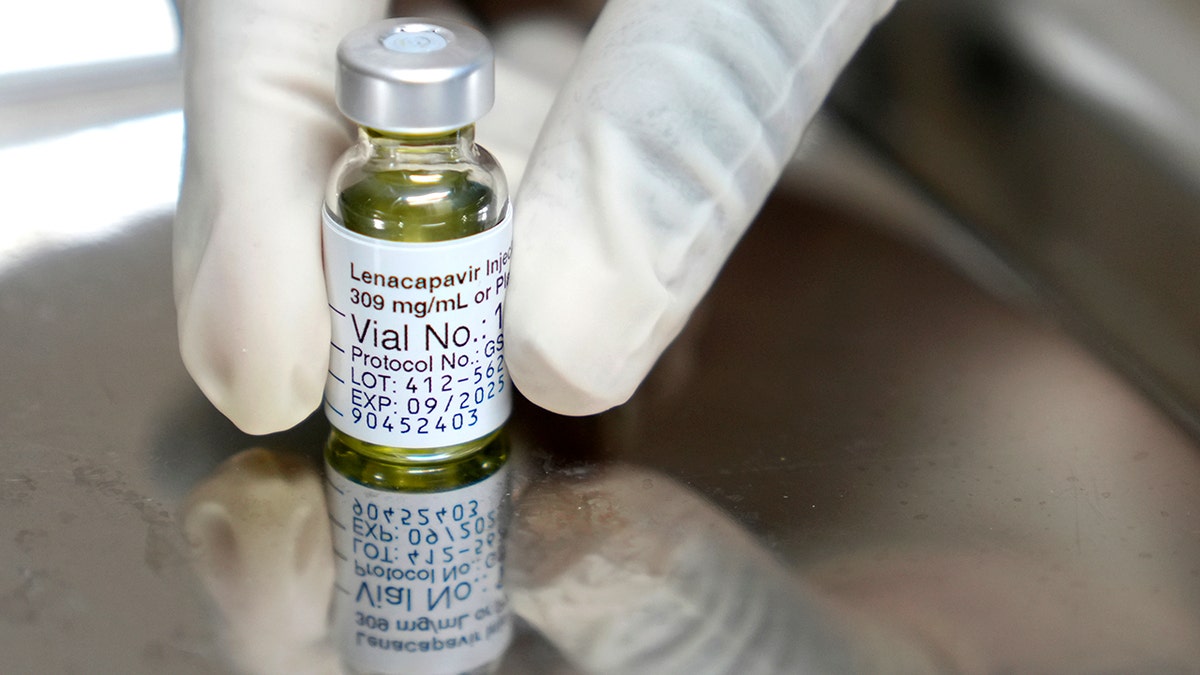


The drug is given as an injectable under the skin that the body then slowly absorbs. Individuals must have a negative HIV-1 test prior to starting the treatment. (AP Photo/Nardus Engelbrecht)
The journal Science named lenacapavir its 2024 “Breakthrough of the Year.”
CLICK HERE TO SIGN UP FOR OUR HEALTH NEWSLETTER
Lenacapavir uses a multi-stage approach that distinguishes it from other approved antiviral medications.



Sold under the name Yeztugo, the company’s injectable HIV-1 capsid inhibitor (lenacapavir) reduces the risk of sexually acquired HIV in adults and adolescents. (iStock)
“While most antivirals act on just one stage of viral replication, lenacapavir is designed to inhibit HIV at multiple stages of its lifecycle,” states the press release from Gilead.
For more Health articles, visit www.foxnews.com/health
“Yeztugo is one of the most important scientific breakthroughs of our time and offers a very real opportunity to help end the HIV epidemic,” O’Day said in the press release.
The most commonly reported adverse reactions during clinical trials included injection site reactions, headache and nausea, according to the company.